Research and Articles
Hotline
- Capital Markets Hotline
- Companies Act Series
- Climate Change Related Legal Issues
- Competition Law Hotline
- Corpsec Hotline
- Court Corner
- Cross Examination
- Deal Destination
- Debt Funding in India Series
- Dispute Resolution Hotline
- Education Sector Hotline
- FEMA Hotline
- Financial Service Update
- Food & Beverages Hotline
- Funds Hotline
- Gaming Law Wrap
- GIFT City Express
- Green Hotline
- HR Law Hotline
- iCe Hotline
- Insolvency and Bankruptcy Hotline
- International Trade Hotlines
- Investment Funds: Monthly Digest
- IP Hotline
- IP Lab
- Legal Update
- Lit Corner
- M&A Disputes Series
- M&A Hotline
- M&A Interactive
- Media Hotline
- New Publication
- Other Hotline
- Pharma & Healthcare Update
- Press Release
- Private Client Wrap
- Private Debt Hotline
- Private Equity Corner
- Real Estate Update
- Realty Check
- Regulatory Digest
- Regulatory Hotline
- Renewable Corner
- SEZ Hotline
- Social Sector Hotline
- Tax Hotline
- Technology & Tax Series
- Technology Law Analysis
- Telecom Hotline
- The Startups Series
- White Collar and Investigations Practice
- Yes, Governance Matters.
- Japan Desk ジャパンデスク
Pharma & Healthcare Update
September 6, 2019Medical Device Round Up: Key Developments in 2019
Regulation of medical devices has speedily evolved in the preceding two years. Before the introduction of the Medical Device Rules, 2017 (“MDR 2017”), the regulation of medical devices and drugs was inextricably linked. In the 18 months since the MDR 2017 has come into force, the biggest challenge has been evolving unique enforcement protocols for regulating medical devices.
In 2019, the Indian Government is stepping up to this challenge. By constituting a special vertical at the apex drug regulatory level, releasing the roadmap for medical devices and bringing a wide variety of medical devices within the regulatory ambit, it is clear that the medical devices industry can expect exciting times ahead.
WIDE CATEGORY OF MEDICAL DEVICES PLACED ON TRACK FOR REGULATIONThe Ministry of Health and Family Welfare (“Health Ministry”) has notified eight additional categories of medical devices on February 8, 2019 under the regulatory framework, to take effect from April 1, 2020.1 The medical devices notified are all implantable medical devices, CT scan equipment, MRI equipment, defibrillators, dialysis machine, PET equipment, X- ray machine and bone marrow cell separators (“Additional Medical Devices”). The Additional Medical Devices have been notified as ‘drugs’ for the purposes of the Drugs and Cosmetics Act, 1940 (“D&C Act”) – the current regulatory framework governing drugs and medical devices. The notification of the Additional Medical Devices comes close at the heels of other categories of medical devices such as nebulizers, blood pressure monitoring devices, digital thermometers and glucometers recently also being notified under the D&C Act (which will come into effect from January 2020). The Health Ministry is also planning on notifying surgical gowns, surgical drapes and incision drapes as medical devices.2
The notification of the Additional Medical Devices marks the government’s strong push towards regulating the medical device sector as a whole. Currently, only 15 categories of medical devices are regulated under the D&C Act through the MDR 2017, which came into effect last year. Prior to the MDR 2017, regulated medical devices were governed by the provisions of the Drugs and Cosmetics Rules, 1945 (“D&C Rules”) – which was originally intended to regulate drugs and cosmetics.
One of the major implications of the notification of the Additional Medical Devices (once it comes into effect) would be the applicability of price control over all the products. Drugs and regulated medical devices are subject to some form of price control under the Drugs (Prices Control) Order, 2013 (“DPCO”), either by restricting importers and manufacturers of drugs/regulated medical devices from increasing the price of the product by more than 10% over the preceding twelve months, or having its ceiling price fixed as determined by the National Pharmaceutical Pricing Authority – the apex drug and regulated medical device pricing regulator.
UNION HEALTH MINISTRY IN THE PROCESS OF INTRODUCING A PATIENT COMPENSATION PLAN FOR FAULTY MEDICAL DEVICESThe B.D. Athani sub-committee (“Sub-Committee”) set up by the Drugs Technical Advisory Board (“DTAB”)- India’s apex advisory body on technical matters relating to drugs- is in the final stages of drawing up a patient compensation plan for faulty medical devices (“Compensation Plan”).3 The Sub-Committee, set up in a DTAB meeting in November 2018 comprises ten persons including senior members of the All India Institute of Medical Sciences, Safdarjung Hospital, State Drugs Controller of Haryana and a representative from the Medical Technology Association of India, among others. The Sub-Committee is headed by B.D. Athani, the Directorate General of Health Services.4
The MDR 2017- the current regulatory framework governing medical devices in India- does not contain any compensation provision for harm caused to patients from faulty medical devices. As a result, compensation in such cases is determined on a case-to-case basis by either the regulatory authorities or the courts. The proposed compensation formula requires (i) the manufacturer/importer of medical device to provide medical management and (ii) compensation as per the New Drugs & Clinical Trial Rules 2019 to the person adversely affected by the medical device.5
Once the Sub-Committee submits its report on the Compensation Plan, it will be considered by the DTAB and then forwarded to the Health Ministry as a DTAB recommendation. Subsequently, the Compensation Plan will become part of the law once the Health Ministry notifies it in the official gazette as part of MDR 2017.
MEDICAL DEVICES TECHNICAL ADVISORY GROUP CONSTITUTED TO ADVISE DRUG REGULATOR ON REGULATION OF MEDICAL DEVICESThe Health Ministry has authorized Central Drugs Standard Control Organization (“CDSCO”)- India’s apex drug regulatory authority- to set up the Medical Devices Technical Advisory Group (“MDTAG”) to advise the CDSCO on the regulation of medical devices.6 The mandate of the MDTAG is to (i) examine issues relating to the implementation of medical device regulation in India and (ii) to make suggestions to the CDSCO for strengthening medical device regulation in the country. The ambit of suggestions made to the CDSCO include:
- Medical device aspect of other Government initiatives such as Make In India,
- Increasing the ease of doing business in India, and
- Taking up matters with the Drugs Consultative Committee and the Drugs Technical Advisory Board- India’s apex advisory committees on drug policy and technical matters- as and when required.
The MDTAG consists of 22 members from different government departments, industry associations and prominent hospitals across the country. The MDTAG will meet with the Drugs Controller General of India (“DCGI”)- India’s apex drugs controller- once every four months and may co-opt persons from other fields as necessary.
India took its first step in medical device regulation in 2017 when the Health Ministry notified the MDR 2017 which came into force on January 01, 2018. The MDR 2017 only applies to medical devices that have been notified by the government. At the commencement of the MDR 2017, only 15 medical devices were notified by the Government while 8 other medical devices (such as blood grouping sera, condoms, surgical dressings and tubal rings) were regulated as drugs. Since then, the Health Ministry has classified 12 Additional Medical Devices bringing the total up to 27 as discussed above.7
ROADMAP FOR MEDICAL DEVICES INTRODUCEDThe DTAB accepted the recommendations of the Health Ministry to regulate all medical devices as ‘drugs’ under the D&C Act.8 Currently, the MDR 2017 governs only 15 medical devices while 8 others were regulated as drugs. After the notification of the Additional Medical Devices, the MDR 2017 will govern 27 medical devices from April 2020.
The need for more comprehensive regulation of all medical devices has been felt for a long time. To that end, the Health Ministry constituted a committee in February 2019 (“MD Committee”) which broadly recommended that medical devices continue to be regulated under the D&C Act. The MD Committee has proposed to regulate all medical devices as drugs in a phase-wise manner as follows:
Phase I
- All manufacturers and importers of non-regulated medical devices should register their devices on a special SUGAM portal. SUGAM is an online portal used to keep an electronic record of all medical device manufacturers and importers in the country along with the medical devices manufactured/imported by them.
- Such registration will be voluntary for up to a period of 18 months after which it will become mandatory.
- The importers and manufacturers must also report a serious adverse event (“SAE”), an event that results in death, hospitalization or injury of a patient, as well as adhere to the Materiovigilance Program of India (“MvPI”) to keep track of medical device SAEs.
Phase II
All low-risk devices (Classes A & B) registered during the preceding 18 month period will be required to obtain licenses within the next 12 months. Registration is required for manufacture, import and marketing the medical device.
Phase III
All high-risk devices (Classes C & D) registered during the preceding 18-month period will be required to obtain licenses within the next 24 months. Registration is required for manufacture, import and marketing the medical device.
The proposed definition of medical devices in the D&C Act includes all medical devices including a software or an accessory, intended by its manufacturer to be used specifically for human beings or animals which does not achieve the primary intended action in or on human body or animals by any pharmacological or immunological or metabolic means but which may assist in its intended function by:
- Diagnosis, prevention, monitoring, treatment or alleviation of any disease or disorder,
- Diagnosis, monitoring, treatment, alleviation or assistance for, any injury or disability,
- Investigation, replacement or modification or support of the anatomy or of a physiological process,
- Supporting or sustaining life,
- Disinfection of medical devices, and
- Control of conception.
Temporary exemption will be provided to the above medical devices notified under the above provision during the 18-month registration period.
The MD Committee also proposed creating a medical device vertical under the CDSCO to bring in additional manpower for regulating medical devices.
The medical device vertical will be created under DCGI and will be headed by an Additional Drugs Controller.
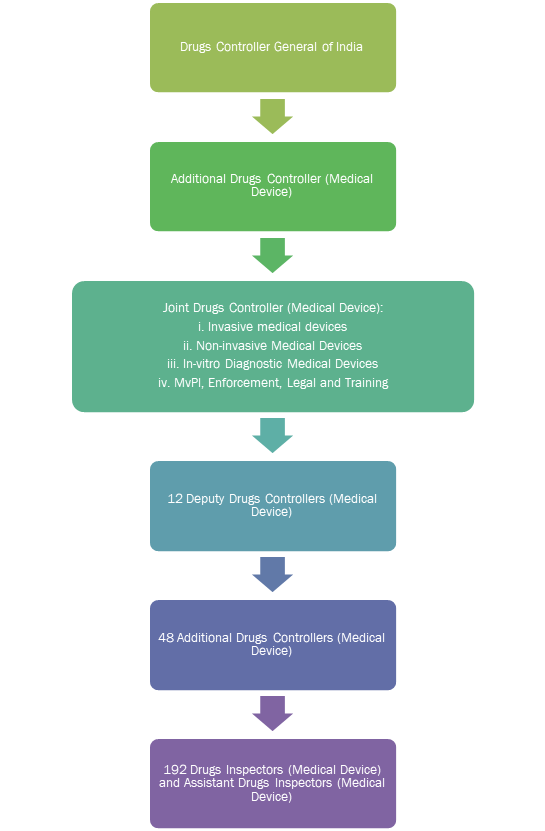
Industry insiders labelled the MD Committee report as a missed opportunity to regulate medical devices separately from drugs. The Medical Technology Association of India has opined that “medical devices are generically different from drugs and cannot be treated as drugs in the long run.” Medical devices require separate regulation for drugs due to the manner in which they are developed, marketed and deployed in a patient by a doctor.9
CONCLUSIONThe regulatory future of medical devices in India was given a definitive direction when the MDR 2017 brought into force at the beginning of last year even though the implementation of the MDR 2017 was progressing at a sluggish pace.
With India’s drug regulatory authority releasing their roadmap for medical device regulation and notifying additional medical devices, it is clear that the Government fully intends to regulate all medical devices within the next two years. The question for the rest of 2019 and beyond is whether continuing to regulate medical devices under the same parent legislation as drugs is sustainable in the long run.
– Shreya Shenolikar, Darren Punnen & Dr.Milind Antani
You can direct your queries or comments to the authors
1 Notice by Central Drugs Standards Control Authority dated May 15, 2019, available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NDM5Ng== (Accessed August 15, 2019).
2 Minutes of 82nd DTAB Meeting held on April 04, 2019 available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=ODc5 (Accessed August 15, 2019).
3 News article on ‘Government readies compensation plan for patients affected by faulty devices’, available at: https://www.livemint.com/companies/news/govt-readies-compensation-plan-for-patients-affected-by-faulty-devices-1564076726975.html (Accessed August 15, 2019).
4 Minutes of 81st DTAB Meeting held on November 29, 2018 available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=NTY2 (Accessed August 15, 2019).
5 Ibid.
6 Office Order by Directorate General of Health Services dated July 22, 2019, available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NDcwNg== (Accessed August 15, 2019).
7 Notice by Central Drugs Standards Control Authority dated May 15, 2019, available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NDM5Ng== (Accessed August 15, 2019).
8 Minutes of 82nd DTAB Meeting held on April 04, 2019 available at: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/common_download.jsp?num_id_pk=ODc5 (Accessed August 15, 2019).
9 News article on ‘The road not taken: a missed chance for medical devices’, available at: https://www.thehindubusinessline.com/specials/pulse/the-road-not-taken-a-missed-chance-for-medical-devices/article26959008.ece (Accessed August 15, 2019).

