Research and Articles
April 4, 2025
2025 Watchlist: Life Sciences Sector India
Introduction
The life sciences sector in India has witnessed a spur in investments and the upward trajectory of investments is expected to continue in 2025. Companies continue to invest in research and innovation which combined with growing government support is offering a strong competitive market for investors. The regulatory developments being adopted by the regulators across sectors are intended to support this trend.
We have captured the regulatory developments to look out for in the life sciences sector in India in 2025 in this update.
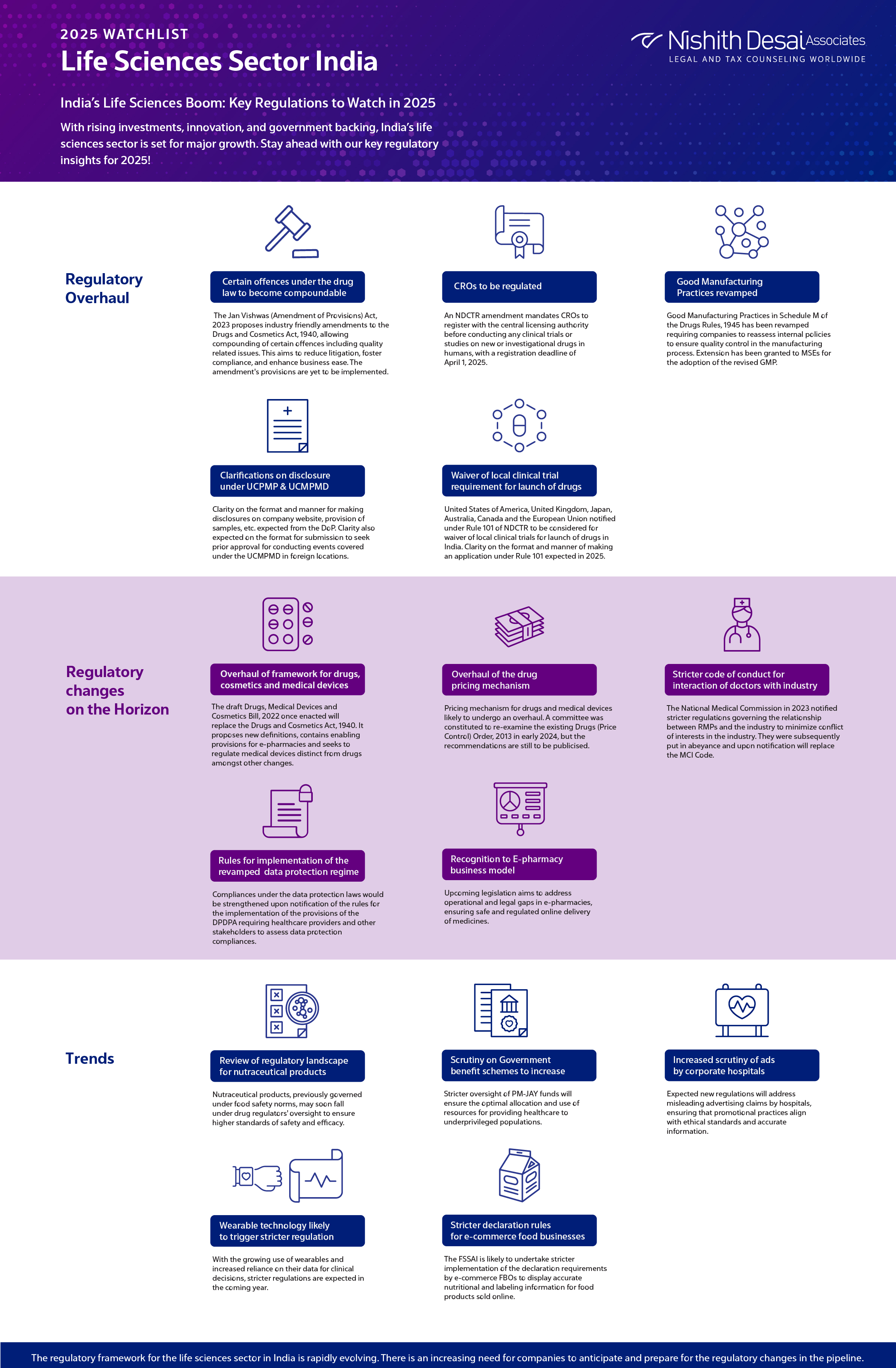
1. Overhaul of framework for drugs, cosmetics and medical devices
The draft Drugs, Medical Devices and Cosmetics Bill, 2022was released for public comments earlier in 2022. Once enacted, the draft bill will replace the Drugs and Cosmetics Act, 1940. It proposes new definitions, contains enabling provisions for e-pharmacies and seeks to regulate medical devices distinct from drugs amongst other changes.
2. Certain offences under the drug law to become compoundable
Amendments proposed to the Drugs and Cosmetics Act, 1940 through the Jan Vishwas (Amendment of Provisions) Act, 2023 will enable compounding of certain offences pertaining to manufacture or sale of drugs in contravention of the act including misbranded, adulterated or spurious drugs, sale of drugs imported in contravention of the act, non-maintenance of records or non-disclosure of information in compliance with the act, etc. It is expected to reduce litigation burdens, foster compliance, and enhance ease of doing business. Provisions of the amendment act are yet to be brought into effect.
3. Overhaul of the drug pricing mechanism
The pricing mechanism for drugs and medical devices likely to undergo an overhaul. A committee was constituted to re-examine the existing Drugs (Price Control) Order, 2013 in early 2024, but the recommendations are still to be publicised. Trade margin rationalisation approach likely to be adopted for drug pricing.
4. Stricter code of conduct for interaction of doctors with the industry to be notified
The National Medical Commission Registered Medical Practitioner (Professional Conduct) Regulations, 2023 (NMC Code) was notified on August 9, 2023, and was subsequently put in abeyance on August 23, 2023 pursuant to representations received from the industry. Once notified it will supersede the Indian Medical Council (Professional Conduct, Etiquette and Ethics) Regulations, 2002. The NMC Code places more stringent restrictions on interactions between RMPs and the industry.
5. CROs to be regulated
An amendment to the NDCTR has introduced a requirement for the registration of CROs with the central licensing authority prior to conducting any clinical trial or bioavailability or bioequivalence study for a new drug or investigational new drug in human subjects. All CROs must be duly registered by April 1, 2025.
6. Good Manufacturing Practices revamped
Good Manufacturing Practices in Schedule M of the Drugs Rules, 1945 has been revamped and made applicable to the manufacturing industry. Revised Schedule M requires companies to reassess internal policies to ensure quality control in the manufacturing process. The deadline for adopting the GMP norms for companies other than MSEs has ended on December 31, 2024. An extension has been granted to MSEs for the adoption of the revised GMP.
7. Clarifications on disclosure requirements under UCPMP and UCMPMD
Nearing the end of the financial year, the industry is likely to receive further clarity on the format and manner of making disclosures on the company website in compliance with the UCPMP and UCMPMD. Further clarity is expected from the DoP on the format for submission of justifications/application to seek prior approval for conducting events covered under the UCMPMD in foreign locations. Clarity is also expected from the DoP on provision of samples, disclosure requirements by companies constituted of various divisions, etc.
8. Six countries to be considered for waiver of local clinical trial requirement for launch of drugs
The drug regulator notified United States of America, United Kingdom, Japan, Australia, Canada and the European Union under Rule 101 of NDCTR to consider waiver of local clinical trials for launch of drugs in India. Further clarity on the format and manner of making an application under Rule 101 expected in 2025.
9. Review of regulatory landscape for nutraceutical products
Amidst growing concern regarding an overlap in the regulation of nutraceutical products by the food and the drug regulator. Recommendations provided by the Inter-ministerial committee constituted for review of regulatory framework surrounding nutraceuticals has recommended to bring such products within the ambit of the drug regulator.
10. Rules for implementation of the revamped data protection regime
Draft of the Digital Personal Data Protection Rules was published earlier this year seeking stakeholder comments. Compliances under the data protection laws would be strengthened upon notification of the rules for the implementation of the provisions of the DPDPA requiring healthcare providers and other stakeholders to assess data protection compliances.
11. Scrutiny on Government benefit schemes to increase
Following high-profile instances of alleged misuse of funds under the PM-JAY scheme, we anticipate heightened scrutiny on hospitals that are empanelled with government schemes. Also anticipate more stringent checks and balances to be put in place.
12. Increased scrutiny of advertisements by corporate hospitals
Following a notice issued by the Supreme Court to the NMC, regulation of advertisements by corporate hospitals is likely to become a reality. Recommendations of the internal panel formed by the NMC in this regard are expected in early 2025 to fill the regulatory void in regulating advertisements and claims made by corporate hospitals.
13. Wearable technology likely to trigger stricter regulation
The past year has witnessed the adoption of the new data protection regime with enhanced compliances surrounding personal data processing and growing sophistication of the Medical Devices Rules, 2017 to keep pace with the technological advancements. Increasing use of wearables and reliance on data generated from such devices to guide clinical decisions is likely to lead to a stricter regulation of such devices in the coming year.
14. Stricter implementation of declaration requirements by e-commerce food business operators
The requirement for display of information such as the declaration of calorific value, allergens, nutritional information, etc. by E-commerce food business operators introduced by the FSSAI is likely to be subject to stricter implementation. E-commerce FBOs not displaying such information upon obtaining the same from the respective FBOs are likely to face action for non-compliance with applicable rules.
15. Recognition to E-pharmacy business model
The report of the CDSCO on the outcome of the stakeholder consultation for the Draft E-Pharmacy Rules as directed by the Delhi High Court is expected in early 2025. The matter is yet to be finally adjudicated. However, with the passage of theDrugs, Medical Devices and Cosmetics Bill, 2022, it is anticipated that the gap in regulation of online pharmacies is remedied.
Takeaway
The regulatory framework for the life sciences sector in India is rapidly evolving. There is an increasing need for companies to anticipate and prepare for the regulatory changes in the pipeline.